Method validation software for clinical laboratories and IVD manufacturers
Assess analytical performance, compare methods, evaluate diagnostic accuracy, and establish reference intervals — all within Excel.
Method validation software for clinical laboratories and IVD manufacturers
Assess analytical performance, compare methods, evaluate diagnostic accuracy, and establish reference intervals — all within Excel.
Trusted by over 60,000 users at top-10 IVD manufacturers for more than 30 years.






-
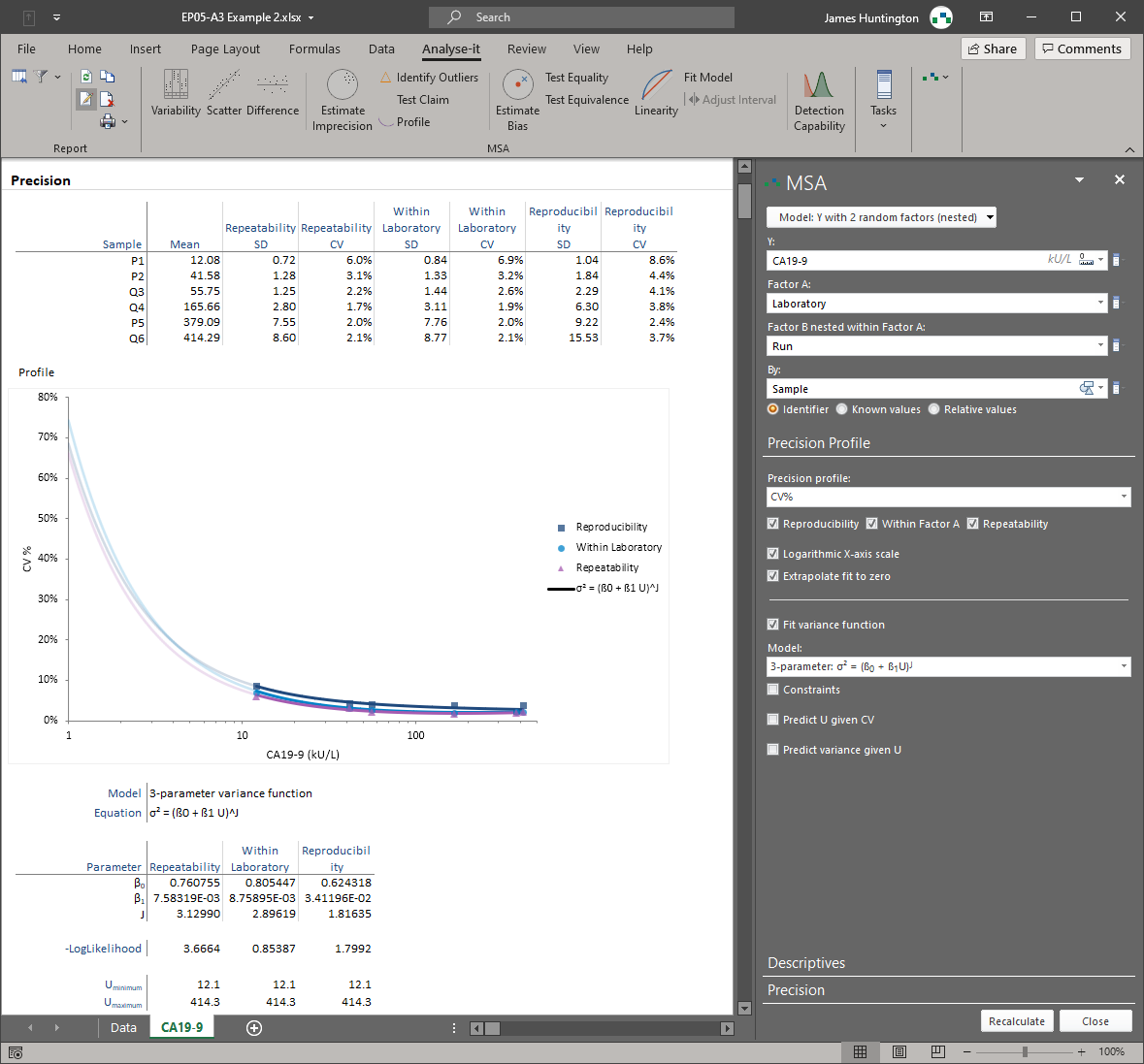
Analytical performance
Analyse‑it helps you verify that your method performs reliably across the full analytical range and meets the requirements for intended use or manufacturer claims. -
Method comparison
Compare methods using Deming, Weighted Deming, Passing–Bablok, OLS, Weighted OLS, and Bland–Altman. Establish real‑world performance, ensure comparability when changing instruments or reagents, and harmonise results across sites or methods. -
Diagnostic test performance
Evaluate diagnostic accuracy with ROC analysis. Assess how well a test discriminates between clinical outcomes, compare multiple tests, and support clinical decision‑making with clear performance summaries. -
Reference intervals
Establish or verify reference intervals for healthy populations. Determine new intervals, verify transferability between laboratories or methods, and partition by relevant factors such as age or sex. -
Implements industry-standard CLSI protocols
Analyse‑it implements 11 CLSI guidelines, including EP05‑A3, EP06‑A, EP09‑A3, EP10‑A3‑AMD, EP12‑A2, EP14‑A3, EP15‑A3, EP17‑A2, EP21‑A, EP24‑A2 (GP10‑A), and EP28‑A3C (C28‑A3C). -
A modern alternative to EP Evaluator
Analyse‑it implements a wider range of CLSI protocols than EP Evaluator and uses the full statistical methods recommended in the guidelines. - Runs in Microsoft Excel for easy deployment across your team. Analyse‑it integrates directly into Excel, letting you import data from instruments, analyse immediately, and generate publication‑ready reports without learning new software or managing proprietary file formats — significantly faster and more flexible than standalone tools.
-
We use Analyse-it frequently for our verification and pre-verification work, in accordance with CLSI guidelines for in-vitro diagnostics. It's saved time and effort compared to the hodge-podge of applications we used before, JMP, SAS, etc...Brian Noland, Ph.D.Principle Scientist, Product DevelopmentBiosite / Inverness Medical Innovations
-
We use Analyse-it for the analysis of data necessary to file 510k. We chose Analyse-it because it works in Excel, includes CLSI protocols, and, unlike EP-Evaluator, lets us analyze data directly from equipment without typing.Thomas D Harrigan, Ph.D.Technical Product ManagerAlfa Wassermann Diagnostic Technologies
-
I used Analyse-It for many product development, product troubleshooting, and technology evaluation activities... your product was the easiest to use, was accurate, and produced publication ready reports.Stanley F. Cernosek, Ph.D.Clinical Chemistry Reagent DevelopmentBeckman Coulter, Inc.
-
Analyse-it has been a tremendous help. I've published and presented at national cardiology meetings and couldn't have accomplished most of my research without it. Using Analyse-it, I even found errors or omissions in the work of our statistician!Regina S. Druz, MD, FACC, FASNCDirector, Nuclear CardiologyNorth Shore University Hospital
-
Although we only scratch the surface of Analyse-it’s capabilities, we have a very high volume of use for the statistics we need. It’s saved us time and the reports look professional.Michael SavageChemistry SupervisorBaptist Hospital East
* Available in the Method Validation and Ultimate editions.
 Start a free 15-day trial! Read more & see examples...
Start a free 15-day trial! Read more & see examples...